Background: The presence and role of follicular T-cell populations other than T follicular helper (Tfh) cells, such as T follicular regulatory (Tfr) and cytotoxic (Tfc) cells, are gaining increasing attention in certain pathological states. However, the ecosystem of follicular T cells in the tumor microenvironment (TME) has not been fully elucidated. In particular, the significance of minor follicular T-cell subsets in the neoplastic follicular environment remains elusive. Here, we aimed to reveal the landscape of follicular T-cell alterations in various cancers, with a particular emphasis on the follicular lymphoma (FL) TME.
Methods: We analyzed single-cell RNA/TCR sequencing data of >500,000 human T cells from FL (obtained from four cohorts) and 25 other cancer types, as well as homeostatic and reactive lymph nodes (LNs), to construct a comprehensive single-T-cell atlas. We investigated differentially expressed genes, RNA velocity, and TCR clonality using this atlas. To determine the functions of neoplastic follicular regulatory (Tnfr) and cytotoxic (Tnfc) T cells, we performed in vitro cytokineproduction and co-culture assays, in combination with cell activation/suppression, cell division, and apoptosis assays, using human FL samples. With the PhenoCycler-Fusion system, we conducted multiplex digital spatial profiling (DSP) of 169 FL samples from two independent cohorts (now being extended to 242 FL samples from three cohorts) for >25 antibodies. We also performed single-cell spatial and protein expression profiling and prognostic analysis.
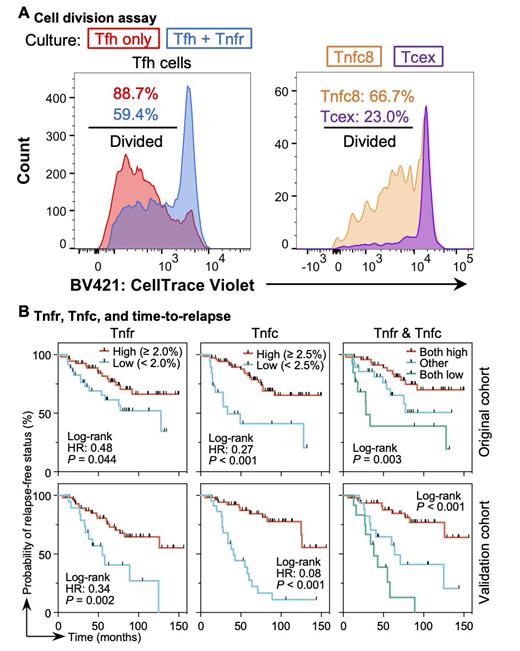
Results: In FL, distinct minor neoplastic follicular T-cell subsets-Tnfr and CD4 (Tnfc4) and CD8 (Tnfc8) Tnfc cells-increased relative to homeostatic LNs. The TCR repertoire analysis revealed that Tnfr cells shared clonotypes with conventional effector regulatory T (Trg) and Tfh cells, whereas Tnfc4 and Tnfc8 cells shared clonotypes with Tfh cells and effector and exhausted (Tcex) cytotoxic CD8 T cells, respectively. In line with these findings, the RNA velocity survey suggested that Tnfr, Tnfc4, and Tnfc8 cells originated from Trg, Tfh, and naïve-like CD8 T cells, respectively. Tnfr and Tnfc cells expressed higher levels of effector genes, including those involved in cytokine release, chemokine response, migration, and PD-1 signaling, than their reactive LN counterparts. The pan-cancer survey revealed that Tfr and CD4 Tfc cells were exclusive to FL, whereas the prevalence and gene expression profiles of CD8 Tfc cells varied across cancers. Tnfr cells were marked by abundant expression of IL10 and IL21, whereas Tnfc cells displayed a unique phenotype, as they concomitantly expressed markers of effector Tfh (e.g., CXCL13, CXCR5, and PDCD1), naïve/stem (e.g., CCR7 and TCF7), central memory (e.g., CD27, CD28, and SELL), and tissue-resident memory (e.g., ITGAE) cells. Hierarchical clustering demonstrated that Tnfc8 cells had transcriptional profiles similar to those of melanoma TCF1 +PD-1 +CD8 + stem-like T cells. DSP of FL detected Tnfr and Tnfc cells frequently localized within and around neoplastic follicles, forming a cellular neighborhood that allowed them to interact closely. Tnfr cells were distributed predominantly near Tfh cells. The functional co-culture assays demonstrated that Tnfr cells suppressed Tfh-cell activation and division, thereby inhibiting Tfh-mediated malignant B-cell activation and survival. Tnfc8 cells showed a higher cell division capability than that of Tcex cells, suggesting that Tnfc8 cells function as a pool of CD8 T cells in neoplastic follicles. The prognostic analysis revealed that Tnfr and Tnfc cell proportions correlated with early disease relapse (i.e., POD24) and predicted a significantly longer time-to-relapse ( P <0.05 for Tnfr and <0.001 for Tnfc cells) in FL. In the multivariate analysis, the prognostic impact of these two cell subsets was independent of the FLIPI. The prognostic analysis findings were confirmed using a validation cohort.
Conclusions: Our multi-omics approach identified the expansion of minor neoplastic follicular T-cell subsets that carry unique transcriptional and functional profiles and robust prognostic impacts. These findings deepen our understanding of the biological and immunological roles of non-Tfh follicular T cells in the lymphoma TME and highlights their clinical potential for patient risk stratification and future therapeutic interventions.
Disclosures
Yoshida:Bristol Myers Squibb: Research Funding; Novartis Pharmaceuticals: Honoraria. Matsue:AstraZeneca: Research Funding. Chiba:Eisai Co., Ltd.: Honoraria; Bayer Pharma: Honoraria; Chugai Pharmaceutical: Honoraria; Kyowa Kirin: Research Funding; Astellas: Research Funding; Thyas: Research Funding. Sakata-Yanagimoto:Mundipharma K.K.: Research Funding; Otsuka Pharmaceutical.: Research Funding; Eisai Co., Ltd.: Research Funding; Kyowa Kirin Co.,Ltd.: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal